CDC Guidelines for Safe Transportation of Vaccines
Transportation of vaccines can be a challenging process. And if not done correctly, it could seriously jeopardize the potency of the vaccines. Vaccines when transported during emergencies or during high demand may be prone to damage due to improper packaging or handling.
Thus, having an emergency plan with clearly specified steps to be taken during emergencies can help health care providers avoid minor or major errors that could potentially compromise the safety and efficacy of the vaccines. These steps can be broken down into three main components – before, during and after transportation.
Before Transportation
The first step towards the safe transportation of vaccines is possessing a sturdy and secure travel container such as a portable medical refrigerator or freezer. Manufacturers’ containers can also be used provided they are not damaged. Conditioned frozen water bottles can be placed inside the transportation unit to help regulate the temperature.
Vaccines must also be secured using insulating material. Bubble wrap, Styrofoam, or packing foam can be placed both above and below the vaccines to form a cushioning layer. Use of digital data loggers can also be utilized to ensure consistent temperatures throughout the travel.
During Transportation
The bottom of the portable medical unit needs to be lined with a single layer of conditioned water bottles. It is important to condition the bottles so that the frozen bottles do not lead to temperature fluctuations. The vaccines need to be placed over these bottles with a layer of insulating material in between. Vaccines are best stored in their original manufacturing boxes until use.
A temperature monitoring device such as a DDL needs to be placed in the center of the unit among the vaccines. After placing some insulating material, another layer of conditioned water bottles needs to be placed in the unit.
Once the vaccines are placed in this specific recommended order, the lid of the unit must be firmly shut with the DDL display and temperature log placed on top of the lid. Ensuring that this particular order of placement is followed will establish the safety of the vaccines placed in the unit. It will also ensure lesser vaccine wastage due to physical damage.
Upon Arrival
After reaching the desired destination, the handler must make a note of the date, time, and temperature within the unit on the temperature log. This information may be useful in case of damages to the vaccine or the possibility of a temperature excursion.
The vaccines need to then be transferred into the storage medical refrigerators immediately after filling the temperature log. In case a temperature excursion is observed or if the vaccines were damaged during transportation, the manufacturer or respective immunization program needs to be contacted immediately for appropriate guidance with troubleshooting.
In the meanwhile, the affected vaccines must not be used but must still be stored as per the required temperatures until a confirmation is received from the appropriate authorities.
Handling of vaccines is a delicate process. The challenges related to safe transportation of vaccines can be overcome through the use of a sturdy yet handy refrigeration unit. The port
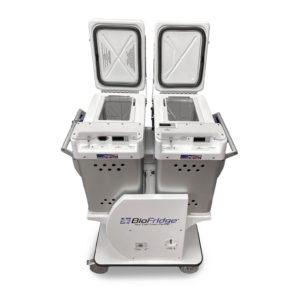
BioFridge 20L x2
able medical refrigerators and freezers at BioFridge can help reduce your burdens when it comes to safe vaccine transportation.
Our portable units are made of stainless-steel hardware and come with a lockable latch to ensure external sturdiness and safety of the unit. They also include digital thermostats that notify the users in case of an excursion. They meet the AABB standards and Code of Federal Regulations. Being an environmentally conscious organization, our units are CFC free and utilize green technology.
We urge you to get in touch with our team by dialing 760-233-8847 or writing to us at sales@biofridge.com so that we can guide you towards your best fit.
Source
https://www.cdc.gov/vaccines/hcp/admin/storage/downloads/emergency-transport.pdf


